Why risk, why now?
All eyes are on Risk Management. Can you manage the scrutiny?
PV teams are relying on passive tools, like spreadsheets and email, to manage their critical processes and commitments. This is even true in risk management, where the stakes couldn’t be higher.
Orbit bridges the gaps left by standard PV systems, connecting your global and local teams. With Orbit, you can share strategies and tools across all of your local markets while monitoring adaptations and deviations in local plans. Only Orbit supports local implementation of additional Risk Minimization Measures in the same seamless interface with your other safety commitments.

Risk Management Plans
Harmonize your global risk management plans.
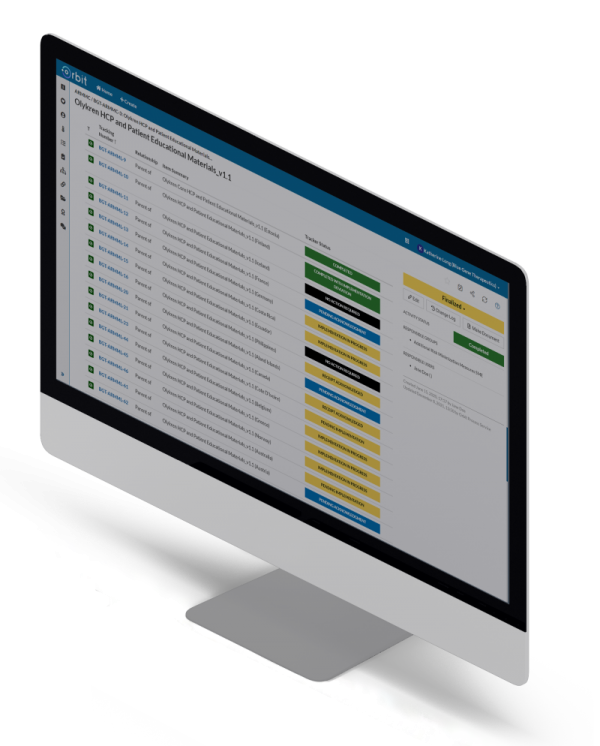
Orbit stores structured RMP details, giving your team total oversight of global variations in safety specifications, commitments, and timelines. With reminders and notifications, staying ahead of your RMP commitments is simple — as is monitoring global progress and implementation metrics.
Demonstrate compliance across all markets with automated, meaningful reports on your global risk management system.
- Core RMP
- dRMP
- REMS (US)
- EU RMP
- Local RMP
- J-RMP
Additional Risk Minimisation Measures
Local implementation.
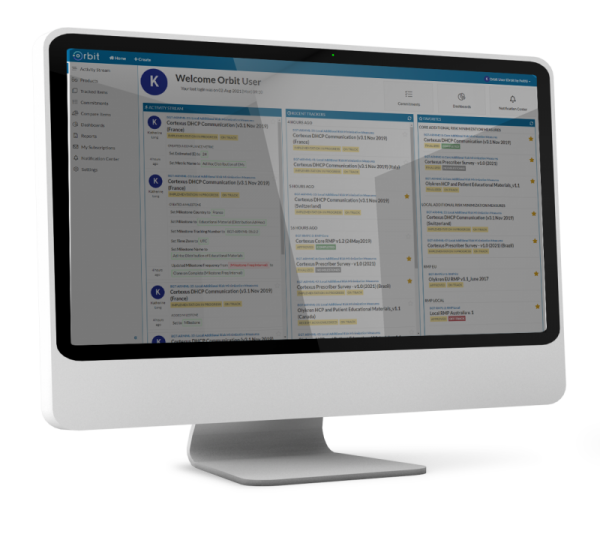
Support local implementation of risk minimization measures like Educational Materials. Orbit gives global safety teams the tools they need to manage a core strategy, guide local safety teams to a successful implementation, and oversee global process metrics.
Only Orbit offers a connected solution to manage RMPs and implement their most challenging and highly regulated commitments.
Manage core implementation plan
Share information and guidance
Oversee local implementation metrics
Blog

Orbit Team Contributes to New Publication on Advancing Pharmacovigilance Practices
New Publication: Tracking a Medicine’s Regulatory Risk Management Commitments Provides

Risk Minimization in the age of AI: preparing for the future of patient safety
Pharmacovigilance is rapidly evolving, driven by technological advancements that transform

Global Coordination, Local Implementation in Orbit: The Industry-Leading Solution for Managing and Tracking Risk Management Activities
Explore how Orbit merges global risk management with local implementation, ensuring safety and compliance worldwide.

Risk Minimization 360: Two Cutting-Edge Technologies That Re-Shape the Way You Manage Your Risk
Explore two cutting-edge platforms – Orbit and Axian One, that enable pharma and biotech companies to achieve a 360-degree view of their risk management activities.

The Revolution of Technology in Pharma
Post-Conference takeaways from DIA Pharmacovigilance and Risk Management Strategies Conference 2023, Bethesda, MD.

Reaching Consensus on Core and Local Implementation
Orbit streamlines risk management by harmonizing core strategies with localized needs, ensuring effective and consistent implementation across all levels.
Connected trackers
Document Exchange
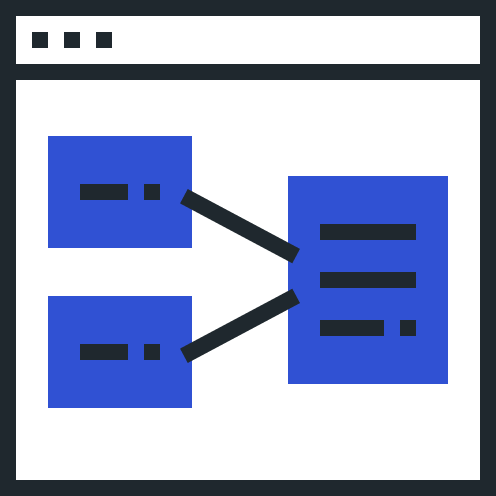
Automatically send updated RMPs to partners based on your Safety Data Exchange and PV Agreements.
Safety Commitments
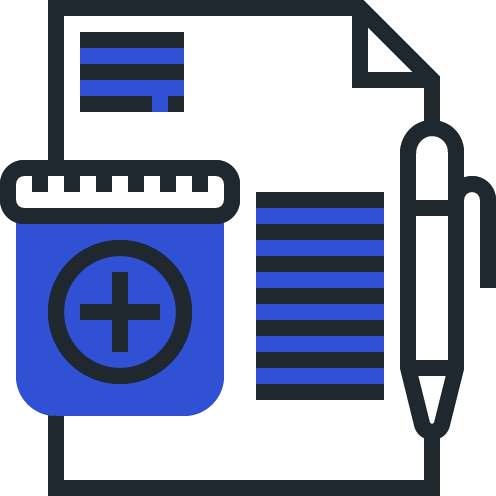
Track Safety Commitments and Health Authority Requests across Regulatory and PV functions.
Areas for Close Monitoring
With integrated MedDRA browsing, manage lists of safety concerns with structured query strategies.
Learn why Orbit is the top choice for Risk Management.
Schedule a call today.