Caught between data retention policies and storage limitations?
Shared inboxes are critical for managing complex and highly-regulated communications in pharma and biotech. This content needs to be retained — often indefinitely– but with cloud storage limitations on email servers, that has proved challenging.
Staying compliant requires a long-term storage solution where companies can archive their emails, freeing up space on their servers and meeting regulatory demands.
Email retention periods for pharmaceutical companies often fall between 5 and 35 years after a product has been removed from the market
The leading provider of SaaS email for the enterprise limits group mailbox storage to 50GB -- once this is met, the inbox will no longer accept new messages

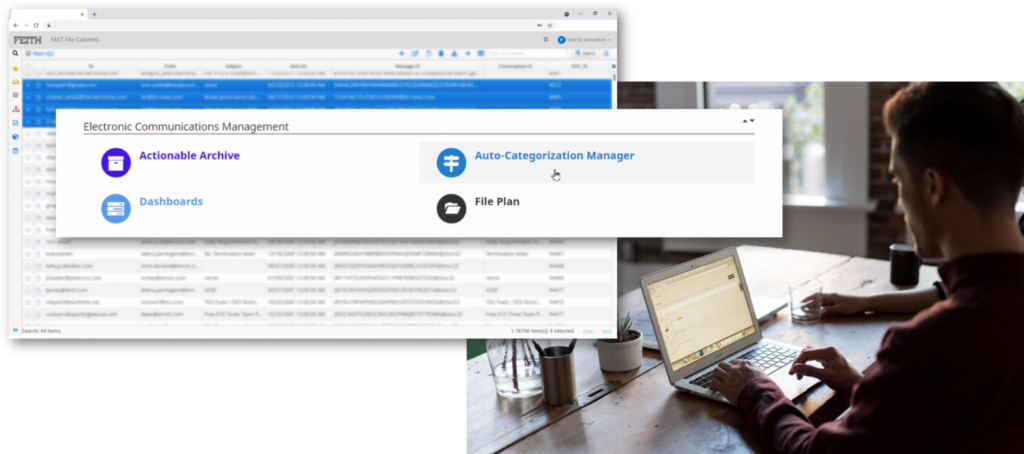
High-volume email storage
Storage and retention, automated.
Mail iQ ingests emails, social media content, and chat from across your organization for long-term, high-volume storage. Using our auto-categorization engine, we can apply retention and disposition rules with no intervention required.
Integrate with all leading email providers
Store emails, attachments, and metadata
Adhere to data retention policies and guidelines
Searchable Archive
Reliable email search for instant access.
No need to rely on your email client to produce content for audits, inspections, or legal requests. Orbit’s Mail iQ is a simple yet powerful tool to demonstrate compliance for both sent and received emails.
Search on metadata, email content, and even attachments:
- Full text search with OCR on attachments and images
- Search by categories and data applied automatically by Feith’s autocategorization engine
- Stand up to large datasets: our archive is equipped for high-speed search even with millions of emails received daily.
It’s simple to find the content you need:
- Reduce search time with quick navigation options and search optimized for large datasets
- View emails in their original format through your desktop application
- Use dashboards to find and report on data in the archive
Once found, place emails and other content into collections:
- Use collections to generate deliverables for audits and inspections
- Place emails on Legal Hold to prevent disposition during legal proceedings
ON-DEMAND WEBINAR
Actionable archiving with Orbit
Safety inbox archiving to manage pharmacovigilance emails and other electronic communications
CHAT AND SOCIAL MEDIA INTEGRATIONS
Regulated communication channels, managed.
Internal messaging tools and public social media accounts are potential sources for highly-regulated data. Like email, this data requires retention, high-volume storage, and could potentially be reviewed during legal proceedings, inspections, and audits.
Mail iQ centralizes email, chat, and social media posts into one archive for end-to-end risk reduction.

Ready to learn more about Safety Mail iQ?
Schedule a call today.
